How to Calculate Heat Capacity: 8 Steps
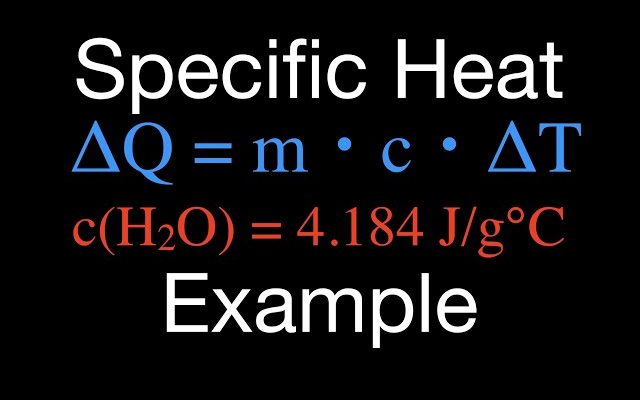
1. Understand the concept of heat capacity: Heat capacity is an extensive property of a substance or object, which represents the amount of heat needed to achieve an increment in temperature by one degree Celsius (or one Kelvin). This property allows us to predict how substances and objects will react when exposed to heat energy. Different materials have different heat capacities, making them suitable for various applications.
2. Know the units: Heat capacity is usually expressed in joules per Kelvin (J/K) or calories per degree Celsius (cal/°C). Different countries may use different units, but both systems express the same concept.
3. Familiarize yourself with the related concepts: Specific heat capacity is an intensive property that represents the amount of heat needed to raise the temperature of 1 kilogram of a substance by one degree Celsius (or one Kelvin). Molar heat capacity is another closely related term which measures the amount of heat required to raise the temperature of one mole of a substance by one degree Celsius (or one Kelvin).
4. Identify the type of calculation you need to perform: Dependingon your problem or experiment, you may have different data available. Therefore, you will need to decide whether you are calculating specific heat capacity, heat capacity for an object or system, or molar heat capacity.
5. Collect and organize your data: Before proceeding with the calculations, gather all relevant data such as mass (m), temperature change (ΔT), heat transfer (q), and molar mass (M) if necessary.
6. Use the appropriate formula:
– For specific heat capacity (c): c = q / (m * ΔT)
– For object/system heat capacity (C): C = q / ΔT
– For molar heat capacity (Cm): Cm = q / (n * ΔT)
Here, n represents the number of moles of the substance.
7. Perform the calculation: Once the appropriate formula is identified, plug in your data and use a calculator to compute the heat capacity. Remember to practice using units consistently throughout your calculation.
8. Interpret and apply the results: Based on the calculated heat capacity, you can understand how substances or objects will behave with changes in temperature and use this information for practical purposes such as selecting materials for insulation or designing more energy-efficient systems.
By following these eight steps, you can successfully calculate heat capacity with confidence, enhancing your ability to predict thermal behaviors and inform decision-making for various applications.


